Background
It is becoming increasingly important in the treatment of MM to improve our understanding of routine clinical practice and of the effectiveness of new agents and regimens outside the clinical trial setting. UVEA-IXA is a European, multicenter, observational, longitudinal cohort study of RRMM pts who received therapy with ixazomib, the first oral proteasome inhibitor (PI), at MM specialist centers via an Early Access Program (EAP) in 8 European countries (Czech Republic, Greece, Hungary, Italy, Slovakia, Slovenia, Spain, UK). Ixazomib was available in Europe via the EAP from Nov 2015, when it was initially approved in the US in combination with lenalidomide and dexamethasone for the treatment of MM pts who have received ≥1 prior therapy, until European approval in Nov 2016. Approvals were based on the results of the phase 3 TOURMALINE-MM1 study (Moreau NEJM 2016). We report data from the second interim analysis of pts enrolled in the UVEA-IXA study.
Methods
The UVEA-IXA observation period comprises two parts: a retrospective chart review, starting from ixazomib therapy initiation in the EAP, followed by a 1-yr prospective follow-up period from the date of chart abstraction, with data captured quarterly per local regulations. Pts eligible for the UVEA-IXA study were adult MM pts in biochemical and/or symptomatic relapse after 1-3 prior lines of therapy, who had not received any anti-MM therapy for >3 cycles (except steroids) at the time of ixazomib therapy initiation, and who had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2. Lenalidomide- or PI-refractory (disease progression on treatment or within 60 d after last dose) pts were excluded from UVEA-IXA. The primary endpoints of UVEA-IXA were response (per International Myeloma Working Group criteria) and progression-free survival (PFS; from ixazomib therapy initiation in the EAP to first documented disease progression/death during the observation period).
Results
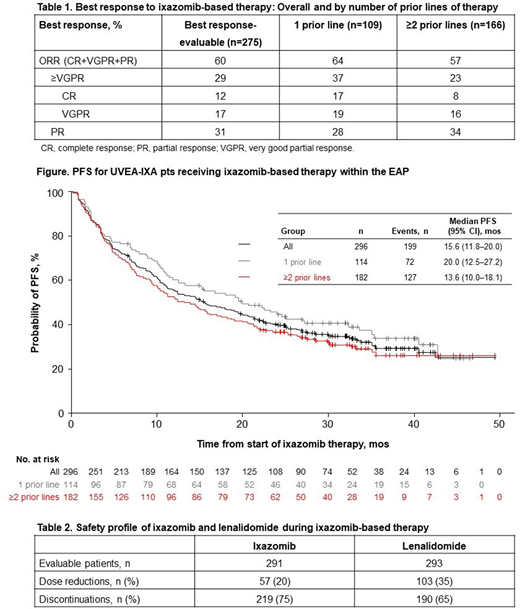
A total of 359 pts were enrolled into UVEA-IXA. At data cutoff (May 22, 2020), 302 pts were evaluable for analysis; 55% were male; median age at enrollment was 68 yrs (range 36-92), with 39% aged ≥70 yrs. At the time of initiating ixazomib therapy, 36/117 (31%) pts had International Staging System (ISS) stage III disease, and 61/301 (20%) had an ECOG PS of 2; 60% of all evaluable pts had ≥1 comorbidity, including hypertension (26%), renal disease (23%), and diabetes (10%). Median time from MM diagnosis was 37.0 mos (range 4.9-231.7), and 39%, 43%, and 18% of pts had received 1, 2, and 3 prior lines of therapy. These included (in any line) bortezomib (89%), thalidomide (52%), transplant (47%), melphalan (22%), lenalidomide (17%), carfilzomib (5%), and pomalidomide (2%). The median follow-up period among 295 pts with available data was 24.9 mos (range 0.2-49.3); of all evaluable pts, 160 (53%) discontinued the study (of these, 88% discontinued due to death). Pts received ixazomib for a median of 10.8 mos (range 0.2-49.3), and most pts also received lenalidomide (97%) and dexamethasone (96%). Among 275 pts with data on best response, the overall response rate (ORR) was 60% (Table 1). Median PFS was 15.6 mos (95% CI 11.8-20.0; Figure). Ixazomib and lenalidomide dose reductions and discontinuations are summarized in Table 2. In 187 pts who received ≥4 cycles of ixazomib, rates of any-grade, grade ≥3, and serious adverse events (AEs) were 60%, 33%, and 23%; the most common AEs of any grade were diarrhea and thrombocytopenia (14% each), rash (7%), peripheral neuropathy (6%), and nausea/vomiting (5%); the most common grade ≥3 AE was thrombocytopenia (6%).
Conclusions
Data from the second interim analysis of UVEA-IXA demonstrate that ixazomib-based therapy is effective outside the clinical trial setting, with an ORR of 60% and a median PFS of 15.6 mos. Compared with TOURMALINE-MM1 pts (ixazomib arm; ORR 78%, median PFS 20.6 mos), UVEA-IXA pts have higher rates of ECOG PS 2 (20% vs 5%) and ISS stage III MM (31% vs 12%), and had received more prior therapies (61% vs 38% had ≥2 prior therapies). The most common AEs were gastrointestinal and hematologic AEs, in line with the well-characterized and manageable safety profile of ixazomib, although data are not directly comparable with clinical trial safety data due to the retrospective/infrequent prospective collection schedule. Ixazomib-based therapy is an effective and tolerable treatment option outside the clinical trial setting.
Ludwig:Celgene: Speakers Bureau; Amgen: Other: Advisory Boards, Research Funding, Speakers Bureau; Takeda: Research Funding; Seattle Genetics: Other: Advisory Boards; Janssen: Other: Advisory Boards, Speakers Bureau; Bristol Myers: Other: Advisory Boards, Speakers Bureau; Sanofi: Other: Advisory Boards, Speakers Bureau. Terpos:Genesis: Research Funding; Sanofi: Honoraria; Genesis: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Janssen: Research Funding; Takeda: Research Funding; BMS: Honoraria; Amgen: Research Funding; Celgene: Honoraria. Mateos:Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees. Boccadoro:Mundipharma: Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kishore:Celgene: Other. Ramasamy:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Oncopeptides: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Fernandez:MediNeos Observational Research: Current Employment. Ferri:MediNeos Observational Research: Current Employment. Bent-Ennakhil:Takeda Pharmaceuticals International AG: Current Employment. Zomas:Takeda Pharmaceuticals International AG: Current Employment. Gavini:Takeda Pharmaceuticals International AG: Current Employment. Hajek:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharma MAR: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Oncopeptides: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal